Science Project on “CARBON DIOXIDE”,. Class 9 & 10 Science Project
Would you like to make Mr. Wizard’s “automatic fountain”? Put a teaspoonful of detergent !the kind that foams) in hot water in a tall jar. Put a lump of dry ice inside the jar. A cloud of foam rises to the top of the jar spills over and creates an amazing fountain that bubbles automatically for a long time.
You can obtain the dry ice from an ice cream man, or perhaps at a local store that sells ice cream.
Be sure that you don’t touch the dry ice with your bare hands. It is so cold that flesh touched by it is quickly frozen. The frozen flesh feels like a burn. Handle the dry ice very carefully. Pick up pieces with a spoon or with tongs. If you put dry ice in a metal pot don’t touch the cold metal or you may get a “burn”. Keep the dry ice in a carton. If you need small pieces the dry ice may be broken by striking it lightly with a hammer.
Put a piece of dry ice into ordinary water (without detergent) in a bowl or pot. A dense white cloud rises from the surface of the water, spills over the sides, flows down to the table and goes over the edge to the floor.
Observe the rapid bubbling around the dry ice in the bowl. Some kind of gas is being released. It rises to the top. The gas is so cold that the moist air above the bowl is condensed to form a cloud because the cloud is very cold it is heavier than normal air and falls to the table.
This is unlike the cloud formed near the spout of a boiling kettle. In that case the heated cloud is warmer than air and therefore rises.
The foam that forms on the top of hot water and detergent is caused by bubbles (gas surrounded by the films of water-detergent mixture when the dry ice was put into the jar containing the water and detergent. it changed to a gas and formed bubbles. Since the gas continued to come up from the dry ice, the bubbles were continuously pushed up and out of the bottle it then looked like an automatic fountain.
Do this with an adult. Light a candle. Let a drop of molten wax fail or, the inside of a metal jar cover. Attach the candle to the cover by pressing its bottom against the molten wax. Put out the flame. Your candle now has a good, safe base for use in experiments.
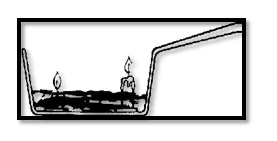
Make another arrangement like this, but with a very short candle. Place the candles inside a pot, the sides of which are much higher than both candles. Light them. Place a piece of dry ice in the bottom of the pot. In about a minute the shorter candle flame is snuffed out. A while later the tall one is snuffed out.
(Note: Whenever you light matches or do experiments with flames be sure to work over the sink to avoid fires. Keep all flammable materials, such as paper and plastic bags, away from the flame.)
It seems as though the gas coming from the dry ice is heavier than air, sinks to the bottom of the pot and puts out flames. The name of this gas is carbon dioxide.
Dry ice is carbon dioxide that has been cooled until it became solid. When you put the very cold, solid carbon dioxide into water it is heated by the water and changes to a gas. This gas bubbles out of the water and goes up into the air. But, because it is normally heavier than air, and is also cold, it tends to fall.
The ability of carbon dioxide gas to put out flames, and its heaviness, make it a very useful fire extinguisher material. One type of fire extinguisher is simply a tank of carbon dioxide under pressure. When the trigger on the tank is pulled the carbon dioxide rushes out and settles down over the flame to smother.
Making Carbon Dioxide
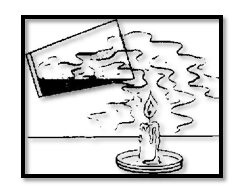
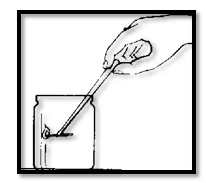
You can easily make carbon dioxide from two simple household “chemicals”. Put a spoonful of bicarbonate of soda in a glass. Pour in a small amount of vinegar. The powder at the bottom of the glass bubbles and forms a froth. While the mixture is bubbling use a pair of tongs to lower a lit match into the glass. The flame goes out.
Make a new batch of froth in another glass. This time “pour” the gas from the glass over a candle flame. It goes out.
The gas termed by the chemical action of bicarbonate of soda and vinegar is heavier than air and puts out flames. It is the same carbon dioxide that was formed by dry ice. This method of making carbon dioxide is used in one type of fire extinguisher.
When the container is turned upside down an acid mixes with bicarbonate of soda to form carbon dioxide. The pressure of the gas then forces the water and some carbon dioxide over the flame to put it out.
Special Fire extinguishers are used for different fires. All fire extinguishers have use labels. Never use old carbon tetrachloride fire extinguishers.
Another way to get carbon dioxide at home is from soda water. Pour some fresh soda water into a glass.
Place the glass in the sink and lower a lit match into it, above the soda water. The flame goes out.
Soda is made by forcing carbon dioxide into water under high pressure. The bottles of soda are tightly capped to prevent the gas from escaping. When you open the bottle the gas bubbles out and starts to escape.
Add a teaspoonful of sugar to the soda. The bubbles of carbon dioxide come out of the water so fast that it forms froth. The same thing hap pens when an ice cream soda is made. In both cases the bubbles come out more rapidly because they have additional surface on which to form. We refer to drinks made from soda water as carbonated because of the carbon dioxide they contain. Carbon dioxide occurs in other places in your home. But in order to find it we need some way to make it reveal its presence.
A Carbon Dioxide Test
Scientists use tests for each substance to discover if it is present in a material. Color, heaviness and appearance, are common tests for solid or liquid materials that we can see. For example, you could recognize iron from its metallic appearance, its heaviness and its ability to be attracted by a magnet.
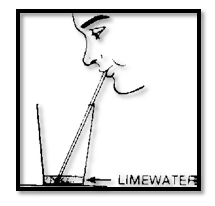
We have seen two tests for carbon dioxide, its heaviness as com- pared with air, and its ability to put out flames. A third test is to mix the gas with limewater. If the limewater turns milky we have very strong evidence that the gas is carbon dioxide.
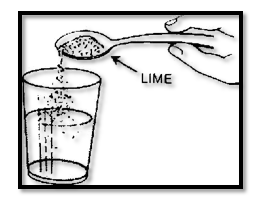
Lime is a white powder that you may get in a drug store or from a chemical set. Perhaps your father has some at home. Put a teaspoonful of lime into a glass of warm water. Mix thoroughly. Cover the glass.
Let the solution remain overnight. The next day pour off the clear solution in the upper part of the glass. This is the limewater for your experiments. Keep it in a small, closed jar.
Pour a small amount of limewater into a pint jar. Throw a match into it and shake the jar. The limewater remains clear.
But then lower a lit match into the jar, using a pair of tongs. Wait until the flame goes out. Remove the burned match. Cover the jar and shake it. The limewater becomes very milky.
The carbon dioxide that causes the milkiness of the limewater comes from the burning of the wood. All fuels, such as coal, wood, oil, and gas contain carbon. The air around us contains oxygen. When the fuel burns the carbon combines with the oxygen and forms carbon dioxide.
Since the carbon dioxide is the product that results from burning. It is chemically changed, and therefore will no longer allow burning. When it settles over a flame it pushes away the oxygen that is needed for burning. And the flame goes out.
Carbon Dioxide and Life
Blow through a straw into a small amount of limewater. Before long the limewater turns quite milky. This shows that there is a great deal of carbon dioxide in your breath Why?
The food that you eat contains carbon. The air around us contains oxygen. The carbon is slowly “burned in all parts of your body and combines with oxygen. The resulting energy supplies you with the heat and motion that you need to live. The carbon dioxide that results from the slow “burning” is a waste that is removed from the body by breathing it out.
But perhaps the carbon dioxide that you found in your breath was already in the air that you breathed in? You can test this possibility by forcing ordinary air through limewater. One way to do this is with a rubber syringe. Place the open end of the syringe tube into a small amount of limewater and squeeze the bulb. Air from the bulb bubbles through the limewater. Remove the syringe andlet it expand back to normal shape.
let it expand back to normal shape. It fills with air again. Put the end of the tube in the limewater again and squeeze the air through the lime water. Do this about 10 times. Little or no milkiness is noticed. Ordinary air seems to have little or no carbon dioxide.In the previous experiment your breath made the limewater quite milky. Your body somehow added carbon dioxide to the air before you breathed it out.
Does the air around us have any carbon dioxide at all? Squeeze air from the syringe 25 times through a very small amount of limewater, no more than a tablespoonful. You may begin to see a slight milkiness. If not, keep doing it until milkiness is noticed. This experiment shows that a small part of the air is carbon dioxide.
Carbon dioxide is a most important substance. Together with water, it is the basic raw material for life. Plants take in carbon dioxide from the air through holes in the bot- toms of their leaves. The roots take in water. The two materials meet in- side the leaf where the energy of sunlight makes them combine to form sugar. This process, known as photosynthesis, is basic to plant life, and is the starting point for production of all of our food.
Plants combine the sugar with minerals taken from the ground to form proteins. These proteins then form the bodies and chemical substances of plants. Animals eat plants and use these materials for their own bodies and for the energy needed for life. Thus, without carbon dioxide our form of life would not be possible.
Scientists think that the amount of carbon dioxide in the air greatly affects the earth’s climate. A small increase in the percentage of car- bon dioxide in the air seems to cause the earth to keep more of the; heat that it gets from the sun. As a result of such an increase the earth has warmed up several degrees during the past 100 years.
It is believed that former ice ages and warm periods were caused by the changes in carbon dioxide in the air.
Today, the burning of fuels for power and heat, in factories, homes and cars is causing an increase in carbon dioxide in the air. Many scientists think that this may cause the earth’s climate to warm up causing many changes for life in the future.
TRY THESE EXPERIMENTS
- Pour some carbon dioxide down a long paper chute toward a lit candle. The chute guides the heavy carbon dioxide gas toward the candle and the flame is put out.
- Light a short candle in a tall pot. Pour soda water around the candle. Carbon dioxide from the soda water puts out the flame.
- Use dry ice to freeze water. Place a small amount of water in a metal cup and place it on a flat piece of dry ice. The water in the cup soon freezes and forms ice.
- Let limewater stand for several days in an open jar. Put an equal amount in a small closed vial. Slight milkiness of the limewater in the open jar is due to carbon dioxide in the air. The closed vial keeps carbon dioxide away from the limewater.
- Put a few drops of vinegar on a piece of chalk. The frothing action is caused by the formation of bubbles of carbon dioxide. A piece of limestone or marble will do the same things. So will baking powder.
- Heat a mixture of flour and yeast in an oven. The flour puffs up and forms bread. When cool, break open the bread and notice the many holes caused by carbon dioxide from the yeast. Baking powder may be used instead of yeast.
- Let a glass of soda stand over- night in the open. In the morning it tastes almost completely “fiat”. The carbon dioxide has escaped into the air. Place another glass of soda in warm water and note that the gas escapes more rapidly.
- Squeeze some lemon juice onto bicarbonate of soda in a glass. The resulting bubbles are carbon dioxide. Any acid will work. Most sour liquids, such as grapefruit juice, are acid and will also do the same thing.
- it’s size. After an hour the piece is much smaller because most of it has gone off into the air Unlike ice, the carbon dioxide has skipped the liquid stage and cone directly to a gas. It is therefore ‘dry’ Ice.
SCIENCE PROJECT
Does dry ice have more cooling ability than ordinary ice?
Measure half a cup of water and pour it into a glass. Do the same with another glass. Put a thermometer in each and measure the temperature.
Weigh a piece of dry ice on an accurate scale. (Be sure that you don’t touch it directly with any part of your body). Weigh an equal amount of chopped ice. Put the dry ice in one glass of water. Put the ice in the other. Stir the water in each glass gently until all of the solids disappear. Read the thermometers. Which one is at a lower temperature?
If you want to make the measurement more accurate find a way to keep both the dry ice and the regular ice under water while they melt.





























Nice information keep up the good work.