Science Project on “Water Tricks”,. Class 9 & 10 Science Project
Water Tricks
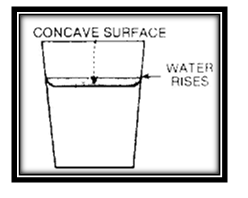
Fill a glass to the brim with water. Place it on a level surface. Drop nails, clips, washers and other small metal objects into the glass of water, one at a time. You would expect some water to spill out every time an object falls in. But nothing of the kind happens. Instead, the water rises higher and higher above the top of the glass. You will be amazed at the number of small objects that can be added to theater before it starts to spill over. The level of water in the glass can get to be about 1/4 of an inch higher than the glass.
Examine the shape of the water surface. Notice that it is convex (bulges outward).Now spill out some water. Notice that the surface of water is concave (curves inward). Observe how the water rises a bit all around the glass. as though it is pulled up by some force.
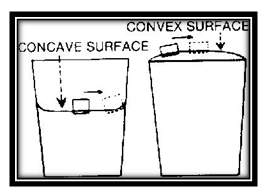
The convex and concave shapes of the water in a glass make it possible for you to do another interesting trick. Ask your friend to float a small cork in the center of a glass of water. Give him the glass and cork and let him get the water to avoid spilling he is sure to leave some space at the top. When he puts the cork into the water it always moves over to the sides, even if placed in the exact center.
You can easily do this trick by making use of the fact that a floating object will always rise to the highest position that is possible.
Fill the glass with water above the brim. The highest point of the con- vex surface is then at the center. The cork floats to that position and remains there.
On the other hand, when the water level is below the top of the glass, the surface is concave and the center is the lowest point. The cork therefore floats away from the center to the higher places near the sides of the glass.
A Water “Skin”
The fact that the surface of the water can hold up a metal boat with holes is explained with the idea of a kind of “skin” on the surface of the water. Such a “skin” would also help explain why the surface bulges outward when the water is made higher than the sides of the glass and curves inward when the water is lower.
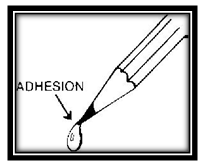
Dip a pencil into water. It comes out wet. In other words water clings to the pencil. The wood attracts the water to itself. This force of attraction is called adhesion.
Now notice the drop of water that forms on the tip of the pencil. The water seems to stick together to form the drop. There seems to be a force of attraction that keeps the water together. This force is called cohesion.
Every material is made up of very tiny bits called molecules. Each molecule of water attracts its neighbors. You see the results of this attraction in the way in which the water bunches together to form drops.
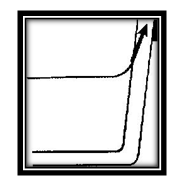
But molecules of water and wood also attract each other. You see the results of this attraction in the adhesion between the pencil and the water that wets it. This attraction explains why the water rises up the sides of the glass to form a concave surface. The molecules of water are pulled up by the attraction of molecules of glass.
Now consider the water alone. The drawing shows a molecule of water (A) beneath the surface of the water. It is being pulled equally by neighboring molecules. Some pull up, others pull down. Some pull to the left, others to the right. The result is that all these forces balance each other and the molecule is rather free to move about inside the water, in any direction at all but now consider molecule (B) at the surface. There are no molecules of water above it to pull upward.
There are air molecules above, but they are relatively far away and exert little force on the water molecules. On the other hand there are many water molecules below and they pull downward strongly. Therefore the molecules at the surface are pulled downward and are squeezed together. This is what causes the tight “skin” that you observed on top of the water. It is called surface tension.
The word “tension’ means “tightening force”. Surface tension therefore refers to the fact that the surface of a liquid is pulled tightly together, by attractions from inside. Water is not the only liquid that shows surface tension. In fact, all liquids show the same effect, some more than others.
You can now see why it is possible for your little metal boat to float. Despite it holes. It takes some force to break the surface tension of the water. The slight weight of the screen was not enough to break through. So it remained afloat Try this experiment. Place the boat on the surface of the water once again.
But this time put some kind of weight, such as a penny, inside the boat. Now the boat sinks. It has been made too heavy and breaks through the surface tension of the water.
Have you ever seen “water bugs” skimming across the top of a pond or stream? They weigh so little that they can be supported by the surface tension of water under their legs.
Again put the boat into water. But this time put it in sideward with the points of the screen piercing the water. Again it sinks. In this case the surface tension was broken by the sharp points and the concentrated weight of the boat on the edge.
Surface tension also helps to ex- plain why you were able to add so many small objects to the full glass of water. The “skin” on the surface of the water acts somewhat like a rubber sheet to keep the water together. Adhesion of the water to the glass attaches this “sheet” to the glass all around the rim.
It is therefore possible to build up a height of water above the rim of the glass without spilling over. This makes the upper surface of water convex.
Have you ever seen “water bugs” skimming across the top of a pond or stream? They weigh so little that they can be supported by the surface tension of water under their legs.
Surface tension plays a very important part in the action detergents (including soap) other cleaning materials.Float your little screen boat once again. Drop a few bits of detergent powder (or soap flakes) into the boat. In a short time the boat sinks to the bottom.
The detergent weakens the surface tension of the water so that it is no longer able to hold up the little boat.
Try this another way. Make up a solution of soap or detergent and water. Try floating your boat in this solution. You now fail to do so. You have weakened the surface tension. This weakening occurs because the molecules of the soap or detergent move between the molecules of water and weaken their attraction for one another. As a result cohesion of the water becomes less and surface tension also becomes less.
Pour some oil on top of a glass of water. Stir it with a spoon to mix the oil and water.
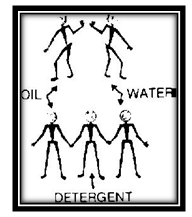
You see the oil gather together in round, flat drops on top of the water. Surface tension causes them to pull together. The round shape is the result of the fact that the liquid in each drop is being pulled inward equally from all sides. Watch the oil. You see the little drops gradually come together and form larger and larger drops. Now add some detergent and mix. This time the oil seems to mix with the water. When oil is poured on water it has a strong surface tension. So does the water. And the adhesion between the oil and water is slight. So, each pulls toward itself and forms its own bunches. And they do not mix.
But when the detergent is added to the oil and water one end of each molecule of detergent is attracted to water molecules. The other end is attracted to oil molecules. The detergent molecule thus forms a kind of bridge of attraction between the two. At the same time the surface tension of both oil and water is weakened and they are pulled together by molecules of detergent.
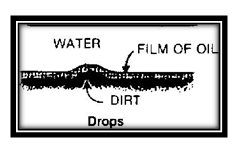
This explains the cleaning action of a detergent. Suppose that your hands are greasy and you wash them with plain water. A film of oil or crease surrounds each bit of dirt. Surface tension of both water and oil keeps the water from pulling the dirt away from your hand.
But with detergent in the water the oil film on the dirt is pulled away and then the water can get at the dirt to wash it away.
Drops
You have already termed drops of oil in water. Now make some drops of water on wax-paper. Use a pencil or medicine dropper to deposit one drop of water on the wax-paper. A round, bulgy drop is formed. Add more small drops of water. They join the first one to form one very large drop. It is round because of the pull of surface tension on all sides. But gravity pulls it down and makes it flatten out.
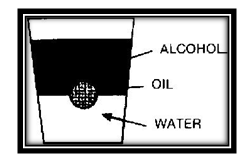
Now try making an almost perfectly round drop, using oil. Add some oil to water in a glass. Then slowly pour rubbing alcohol down the sides of the glass. The alcohol floats on top of the oil and water. The buoyant effect of the alcohol on the oil tends to cancel out the flattening effect of gravity.
Now the oil drop appears to be an almost perfect sphere.
Have you ever wondered how glass marbles are made in the shape of small spheres? Blobs of molten glass are dropped from the top of a tall tower. As each blob of glass falls, surface tension pulls the molecules toward the center to give it a spherical shape. By the time the molten glass has reached the bottom of the tower it has hardened in to a solid, ball-shaped marble. BB shot is made in the same way from molten lead. Scientists have learned a great deal from the study of surface tension. This study has led to important practical results in the manufacture of cleaning materials and chemicals. But equally important is the knowledge about atoms and molecules that have come from this study. This knowledge has been put to use in many fields of science.
TRY THESE EXPERIMENTS
- Tie the ends of a 6-inch silk thread to form a loop. Place the loop in water. Touch the inside of the loop with a soapy toothpick. Surface tension is weakened inside the loop and the surface tension outside pulls the thread into a circle.
- Sprinkle fine talcum powder (baby powder) on water in a dish. Touch a soapy toothpick to the powder. The surface tension weakens at that point and you see the powdered water suddenly pulled away in all directions by the stronger surface tension elsewhere.
- Make two cloths dirty by rubbing with grease or oil. Wash one with warm water and detergent. Wash the other with warm water. Molecules of detergent help the water molecules pull the dirt away from the cloth and make it much cleaner.
- Use a rubber band to fasten a double layer of cheese cloth over the open end of the bottle. Pour in water through the cheese cloth. Invert the bottle. Surface tension and air pressure combine to keep the water from spilling out.
- Punch some holes in the side of a can. Let water stream out of the holes. Pinch the streams together with your fingers. Surface tension makes them form one stream
- Blow up a bubble. Before It comes off the pipe stop blowing. Surface tension gives the bubble its spherical shape. Watch how it also pulls the bubble together and makes it slowly shrink.
- Make “sheets” by letting water from the faucet strike a spoon or knife. Surface tension keeps the water together so as to form the sheet. Many interesting patterns may be made in this way.
- Float a needle or razor blade on the surface of water. Gently lower it flat onto the water. Surface tension of the water keeps it afloat.
- Dip a matchstick or toothpick onto a bar of soap. Place it in water in a sink or bowl. The soap breaks the surface tension and the match is pulled away by the surface tension at the other end. Try dipping the mater in different household cements and glues. Do these with adult supervision.
- Float two matchsticks in water about one inch apart. A drop of alcohol falling between the match sticks weakens the surface tension and makes the matchsticks fly apart.
- Spray or pour some water onto a dusty screen or floor. Tiny, round droplets form because of surface tension. The dust keeps the water from flattening out and wetting the wood.
- Tie three strings to a can cover Tie a rubber band to the strings. When you try to pull the cover away from the surface of water the rubber band stretches because of the attraction between the water and the can cover.
SCIENCE PROJECT
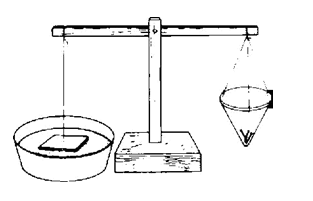
The force of surface tension can be measured, You will need a balance device like the one shown in the diagram. You can make one from wood with a pin as a pivot.
Cut a two-inch square of flat plastic. Put a tiny hole in the center with a needle. Tie a knot in a thread and use it to suspend the plastic from one end of the balance. Use very small nails and bits of paper on the other end to balance the plastic.
Bring a pan of water under the plastic until the plastic touches the water. Now add tiny weights (small nails) to the pan on the other side until the plastic suddenly breaks away from the surface of the water. The extra weight required to do that is a measure of the surface tension of the water, the force with which It attracts the plastic.
To find the amount of weight. balance small nails against a nickel. A nickel weighs 5 grams. If 50 nails balance a nickel, then each nail weighs (5 grams)/50 or 1/10-gram.